AMG group research field:
CHEMICAL ABSORPTION IN INTERMETALLIC COMPOUNDS
VOLUMETRIC TECHNIQUE
Principle: Measuring of hydrogen pressure drop due to absorption of hydrogen in sample.
Equipment (self-constructed): Quartz tube containing a weighed quantity of sample powder, thermostated at a given temperature using a tube furnace. Connection with vacuum and hydrogen reservoir is realized using a set of valves.
Performance: working pressure: 1 bar; temperature range: 300-1000K; maximal measurable pressure change in the system (due to gas absorption in sample): (100.00 ± 0.01) mbar.
Absorption capacity calculation: Calculation of maximal hydrogen absorption i.e., number of hydrogen atoms absorbed per metal atom, H/M, is based on the equation of ideal gas state, PV = nRT, [P – measured hydrogen pressure drop (Pa); working, hydrogen, volume (m3); n- number of hydrogen moles; R – gas constant (J/K mol); temperature (K)]
Hydriding kinetics: Based on measured H/M change during time, kinetic parameters are determined. Some examples are given in references below.
The use of the equipment is free of charge, but it requires the presence of an authorized scientist. All participants must apply for user time in advance.
For more information refer to Dragica Stojić and/or Katarina Ćirić
References:
1. Dragica Lj. Stojić, Sandra V. Kumrić, Jelena N. Belošević-_Čavor, Jana S. Radaković, Božidar Dj. Cekić, Slavko V. Mentus
Hydridic, thermodynamic and kinetic properties of Hf2Ni intermetallic phase
International Journal of Hydrogen Energy, 34 (2009) 3764-3770.
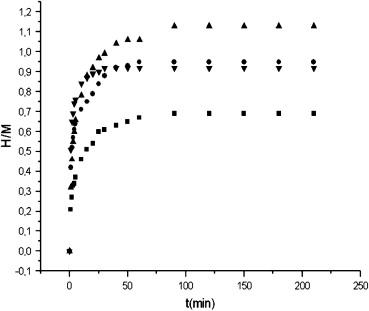
The hydrogen absorption isotherms of Hf2Ni at various temperatures: ■ 300 °C, ● 400 °C, up triangle, filled 450 °C, and down triangle, filled 550 °C.
2. Dragica Lj. Stojić, Sandra V. Kumrić, Tomislav D. Grozdić, Vasil J. Koteski, Božidar Ð. Cekić
Hydrogen production and storage – Investigation of Hf – based intermetallics
Journal of Power Sources, 193 (2009) 165–169.
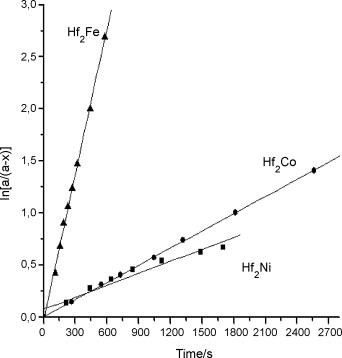
The dependence of ln[a/(a − x)] vs. time at 573 K; a is equilibrium (maximum) amount of H/M (hydrogen atoms absorbed per one molecule of alloy) and x is H/M for time t.
3. Conić, D., Gradišek, A., Radaković, J., Iordoc, M., Mirković, M., Čebela, M., Batalović, K.
Influence of Ta and Nb on the hydrogen absorption kinetics in Zr-based alloys
International Journal of Hydrogen Energy, Volume 40, Issue 16, 4 May 2015, Article number 15498, Pages 5677-5682
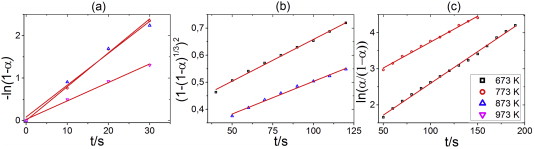
The dominant reaction mechanisms in the first (a), and second (b), (c) reaction stage in the final cycle of hydrogen absorption by Zr–12Ta sample in the temperature range 673–973 K
4. V. Koteski, J. Belošević-Čavor, K. Batalović, J. Radaković, A. Umićević
Hydrogen diffusion in MgH2 doped with Ti, Mn and Fe
RSC Advances 5 (2015), p. 34894